Coronavirus Vaccines In Trouble As Participant
In Research Trial Becomes Seriously Ill Confirming Previous Site
ClaimsSeptember 10. 2020

AstraZeneca
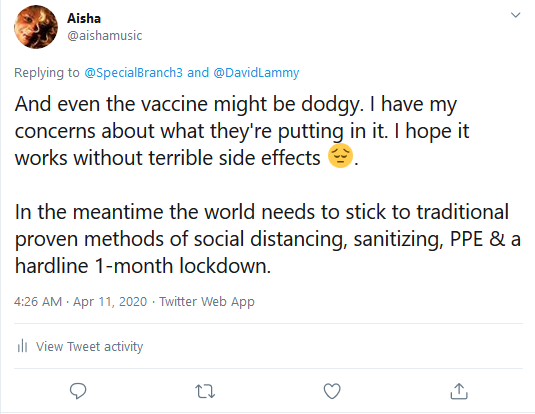
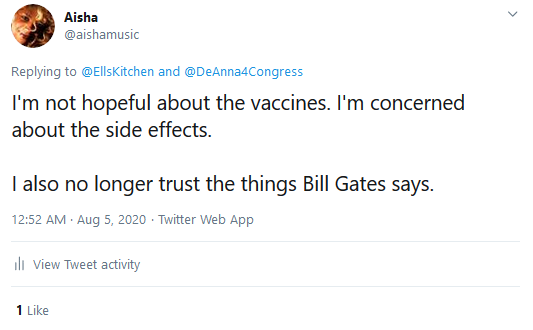
I stated on this site and the social networking
website Twitter in time stamped items the rushed coronavirus vaccines currently
in production pose health risks (see time stamped items below that I
wrote beginning in April of this year).
This week I was proven right. AstraZeneca has paused a phase 3 clinical trial of
their coronavirus vaccine due to a participant becoming "seriously
ill." The participant that became ill has been hospitalized and not
much information is available on their condition.
America, Britain, Canada, Germany, China and Russia
have all been working on vaccines. However, Dr. Anthony Fauci stated there will not be a
coronavirus vaccine this year. The World Health Organization
expressed they are not of the belief there will be a coronavirus
vaccine this year.
Several prominent scientists are casting doubt on the Russian
government's claims they have produced a working vaccine. Russian
President Vladimir Putin has made statements regarding his
government's vaccine being administered to Russians, even his family
members, and he states it works. However, Putin has not released any
scientific data on his government's vaccine for peer review or any
form of scientific evaluation.
My time stamped tweets on that have been proven correct on the
subject:



STORY SOURCE
COVID-19 vaccine trial halted after participant becomes
seriously ill
Published 1 day ago - ATLANTA - The drug giant
AstraZeneca has temporarily shut down its coronavirus vaccine trial,
after a British volunteer in the study became seriously ill. A
company spokesperson called the pause "routine," and said it is
something that needs to happen anytime a participant in a clinical
trial suffers an unexplained severe adverse reaction...
AstraZeneca has not revealed what sort of medical
complication the trial participant experienced. Until more is known
about this case, Dr. del Rio, whose hospital system is testing the
Moderna mRNA vaccine, another leading COVID-19 vaccine candidate,
says it makes sense for AstraZeneca to halt its research to
investigate the issue...
https://www.fox5atlanta.com
Fauci says a coronavirus vaccine is ‘unlikely’ by U.S.
election
Published Tue, Sep 8 20201:49 PM EDTUpdated Tue, Sep
8 20202:53 PM EDT - The CDC has asked states to ready facilities to
distribute a coronavirus vaccine by Nov. 1. Dr. Anthony Fauci said
at a health conference that it’s more likely a vaccine will be ready
by “the end of the year.”
White House coronavirus advisor Dr. Anthony Fauci
said Tuesday a coronavirus vaccine probably won’t be ready by the
U.S. presidential election even as the Centers for Disease and
Prevention asks states to ready distribution facilities by Nov. 1.
At a health conference, Fauci said it’s more likely
a vaccine will be ready by “the end of the year” as drug companies
Moderna and Pfizer race to complete patient enrollment for their
late-stage vaccine trials by the end of September.
“It’s unlikely we’ll have a definitive answer” by
the Nov. 3 election, the director of the National Institute of
Allergy and Infectious Diseases said at the Research! America 2020
National Health Research Forum.
https://www.cnbc.com
Scientists Cast Doubt on Results From Russian Covid Vaccine
13 hrs ago - (Bloomberg) -- A group of international
scientists questioned results from a study of Russia’s fast-moving
coronavirus vaccine that were published in the Lancet medical
journal, saying some of the findings appeared improbable.
The researchers flagged concerns over seemingly
identical levels of antibodies in a number of study participants who
were inoculated with the experimental vaccine. This and other
patterns in the data present “several different points of concern,”
according to an open letter written by Temple University professor
Enrico Bucci and signed by more than a dozen other scientists.
The Lancet published results of the early-stage
trial last week, offering the first look at the Russian study to be
vetted by outside experts. A move by the government to approve the
shot for use based on the initial results had drawn widespread
skepticism, since vaccines aren’t normally cleared before broad
assessments of their efficacy and safety.
The Lancet said it encourages debate on papers that
it’s published. “We have shared the letter directly with the authors
and encouraged them to engage in the scientific discussion,” the
journal said in a statement...
https://www.msn.com
RELATED ARTICLES

